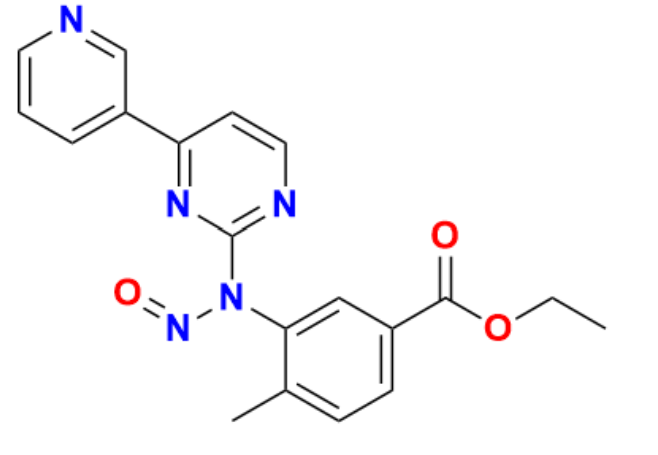
Nilotinib hydrochloride, due to its significance as a targeted cancer therapy, has significantly improved the outcomes for millions of people all over the world. The scope of its function, however, extends far beyond oncology wards and into laboratories that are developing new treatments. This comprehensive guide, which was produced by Qingmu Pharmaceutical, an established manufacturer, investigates the revolutionary qualities of nilotinib as well as its cutting-edge manufacturing process.
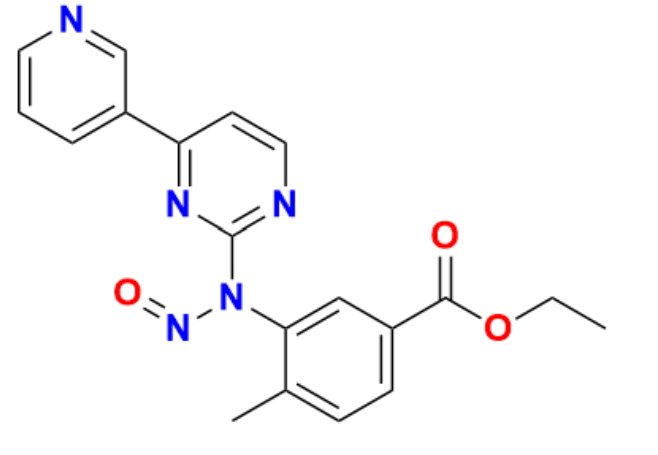
Nilotinib Hydrochloride: Applications and Applications
Through the process of kinase inhibition, nilotinib hydrochloride is able to inhibit the proliferation of cancer cells by binding to the ATP-binding site of tyrosine kinase enzymes. One of its primary applications is in the treatment of chronic myeloid leukemia (CML), where it selectively inhibits the BCR-ABL fusion proteins that are responsible for the disease. On the other hand, the properties of nilotinib can also be applied to other pathologies:Blocks KIT and PDGFRα kinases, which are responsible for fueling specific subtypes of gastrointestinal stromal tumors (GIST) that are difficult to treat. FMS-like tyrosine kinase 3 (FLT3) mutations, which are responsible for up to thirty percent of acute myeloid leukemia, have been found to be effective against the disease. The potential to inhibit PDGFR, CSF1R, DDR1, and other kinases that have been implicated in sarcomas, gliomas, and other types of solid tumors has been demonstrated on solid tumors. Nilotinibs are used by researchers for a variety of purposes, including but not limited to oncology, receptor tyrosine kinase interactions, and the optimization of analog derivatives. Because of its well-understood pharmacology, preclinical safety and efficacy research is also supported by it. In general, nilotinib hydrochloride is an extremely valuable research and development tool, in addition to its clinical benefits.
The Production of Nilotinib Hydrochloride Product
The manufacturing of nilotinib hydrochloride by Qingmu Pharmaceutical is accomplished Nilotinib manufacturer through the utilization of a proprietary process that optimizes quality, yields, and sustainabilityA critical multi-step asymmetric synthesis is responsible for the formation of nilotinibs, which consist of a trifluoromethylphenyl ring and a pyrimidine core. This is accomplished through a series of nitroration, reduction, and coupling reactions. The separation of nilotinib from residual byproducts and impurities is accomplished through column chromatography, which also allows for the recovery and recycling of valuable solvents. The stable monohydrate crystalline form can be achieved through crystallization through the application of current good manufacturing practices (cGMP), which require the strict regulation of temperature, addition of solvent, and stirring. Drying and milling: Nilotinib's critical quality attributes are preserved through the use of vacuum drying due to the absence of thermal degradation. Through micronization, bioavailability is increased.
Characterization
Extensive analyses have demonstrated that nilotinib hydrochloride satisfies stringent purity, identity, and residue standards that have been established by compendial monograph specifications. LDPE bags are used to package nilotinib, which is then placed inside drums that have their humidity and temperature under control. This is done in accordance with the ICH guideline for the packaging of pharmaceutical products.
Certifications: Qingmu Pharmaceuticals' facilities, quality management systems, and environmental practices are all in compliance with international regulatory standards. These facilities are WHO GMP compliant. They have optimized their route to produce 99% pure nilotinib hydrochloride monohydrate API in commercial batches that are in compliance with pharmacopeial standards. This is accomplished through the use of advanced continuous processing. Supply that is both reliable and secure is maintained by stringent quality oversight.
Purchasing from Qingmu Pharmaceutical Offers Numerous Advantages
Within the realm of nilotinib API active pharmaceutical ingredient (API) development and bulk purchasing, Qingmu Pharmaceutical stands out as a preferred strategic supplier:In terms of mass production scalability, multi-ton capacity is capable of reliably and without delay satisfying the requirements of even the largest commercial-scale consumers. Because of the scale, efficiency, and direct sales model, the prices are at least 15-30% lower than the average for the market. This results in cost savings for bulk purchases. Over the course of several decades, the company has maintained a singular concentration on pharmaceutical synthesis, which has resulted in an unrivaled level of Nilotinib manufacturer and process optimization experience. Dossier submission support, successful FDA/WHO pre-approval site inspections, and ISO certifications are all essential components of regulatory experience that contribute to the development of client confidence. For the purpose of accelerating projects, technical development services include not only the development and validation of analytical methods but also feasibility studies and small-scale synthesis. Manufacturing under ICH, FDA, and EMA cGMPs with full traceability and pharmacopoeial compliance and quality control standards that are guaranteed to be in accordance with quality assurance standards.
Expertise in Logistical Operations
This strategic location in Chengdu enables express deliveries anywhere in the world within two to five days, as well as damage insurance and shipment tracking through major carriers.
Nilotinib hydrochloride active pharmaceutical ingredient (API) requirements of manufacturing companies and researchers all over the world are easily met by Qingmu Pharmaceutical, which possesses the capabilities, knowledge, and certifications that define industry leadership. They are able to achieve the highest possible standards throughout the entire process of product development, manufacturing, and supply thanks to their partnership. Whether it is being used in clinical trials to treat chronic myelogenous leukemia and other aggressive cancers or is still in the early stages of development to address novel therapeutic areas, the manufacturer of nilotinib API continues to break new ground. Keeping a consistent supply of API of the highest possible quality becomes an absolute necessity as its significance increases. Qingmu Pharmaceutical is considered to be the ilotinib hydrochloride API source partners that can be relied on unconditionally to power innovation on a global scale. This is due to the company's unparalleled manufacturing expertise and value-added services that comply with cGMP standards.